Interaction of an electron beam with a sample target produces a variety of emissions, including x-rays. An energy-dispersive (EDS) detector is used to separate the characteristic x-rays of different elements into an energy spectrum, and EDS system software is used to analyze the energy spectrum in order to determine the abundance of specific elements. EDS can be used to find the chemical composition of materials down to a spot size of a few microns, and to create element composition maps over a much broader raster area. Together, these capabilities provide fundamental compositional information for a wide variety of materials.
How it Works - EDS
EDS systems are typically integrated into either an SEM or EPMA instrument. EDS systems include a sensitive x-ray detector, a liquid nitrogen dewar for cooling, and software to collect and analyze energy spectra. The detector is mounted in the sample chamber of the main instrument at the end of a long arm, which is itself cooled by liquid nitrogen. The most common detectors are made of Si(Li) crystals that operate at low voltages to improve sensitivity, but recent advances in detector technology make availabale so-called 'silicon drift detectors' that operate at higher count rates without liquid nitrogen cooling.
An EDS detector contains a crystal that absorbs the energy of incoming x-rays by ionization, yielding free electrons in the crystal that become conductive and produce an electrical charge bias. The x-ray absorption thus converts the energy of individual x-rays into electrical voltages of proportional size; the electrical pulses correspond to the characteristic x-rays of the element.
Strengths
- When used in 'spot' mode, a user can acquire a full elemental spectrum in only a few seconds. Supporting software makes it possible to readily identify peaks, which makes EDS a great survey tool to quickly identify unknown phases prior to quantitative analysis.
- EDS can be used in semi-quantitative mode to determine chemical composition by peak-height ratio relative to a standard.
Energy-Dispersive X-Ray Spectroscopy. Energy-dispersive X-ray spectroscopy (EDX) is a surface analytical technique where an electron beam hits the sample, exciting an electron in an inner shell, causing its ejection and the formation of an electron hole in the electronic structure of the element. EDS - Energy Dispersive X-Ray Spectroscopy from the Technology Data Exchange - Linked to trusted TDE listed vendors.
- The subject of this project is about 'Energy Dispersive X-Ray Fluorescence ' (EDXRF). This technique can be used for a tremendous variety of elemental analysis applications. It provides one of the simplest, most accurate and most economic analytical methods for the.
- Energy-dispersive X-ray spectroscopy (EDX or EDS) is an analytical technique used to probe the composition of a solid materials. Several variants exist, but the all rely on exciting electrons near the nucleus, causing more distant electrons to drop energy levels to fill the resulting 'holes.'.
- Dispersive X-ray spectroscopy (WDS or WDX) separates the X-rays by diffracting them with crystals, collecting one wavelength, or energy, at a time. In contrast, its sister technique, energy- dispersive X-ray spectroscopy (EDS or EDX), collects X-rays of all energies simultaneously. The two methods are almost always used in combination.
Limitations
- There are energy peak overlaps among different elements, particularly those corresponding to x-rays generated by emission from different energy-level shells (K, L and M) in different elements. For example, there are close overlaps of Mn-Kα and Cr-Kβ, or Ti-Kα and various L lines in Ba. Particularly at higher energies, individual peaks may correspond to several different elements; in this case, the user can apply deconvolution methods to try peak separation, or simply consider which elements make 'most sense' given the known context of the sample.
- Because the wavelength-dispersive (WDS) method is more precise and capable of detecting lower elemental abundances, EDS is less commonly used for actual chemical analysis although improvements in detector resolution make EDS a reliable and precise alternative.
- EDS cannot detect the lightest elements, typically below the atomic number of Na for detectors equipped with a Be window. Polymer-based thin windows allow for detection of light elements, depending on the instrument and operating conditions.
Results
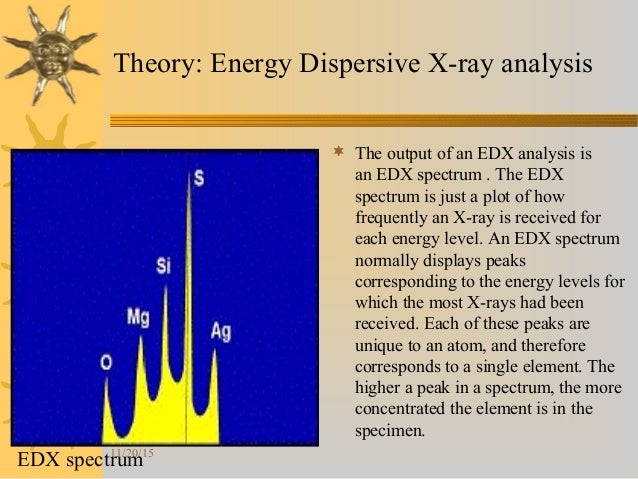
A typical EDS spectrum is portrayed as a plot of x-ray counts vs. energy (in keV). Energy peaks correspond to the various elements in the sample. Generally they are narrow and readily resolved, but many elements yield multiple peaks. For example, iron commonly shows strong Kα and Kβ peaks. Elements in low abundance will generate x-ray peaks that may not be resolvable from the background radiation.
Energy Dispersive X-ray Spectroscopy Pdf
References
- Severin, Kenneth P., 2004, Energy Dispersive Spectrometry of Common Rock Forming Minerals. Kluwer Academic Publishers, 225 p.--Highly recommended reference book of representative EDS spectra of the rock-forming minerals, as well as practical tips for spectral acquisition and interpretation.
- Goldstein, J. (2003) Scanning electron microscopy and x-ray microanalysis. Kluwer Adacemic/Plenum Pulbishers, 689 p.
- Reimer, L. (1998) Scanning electron microscopy : physics of image formation and microanalysis. Springer, 527 p.
- Egerton, R. F. (2005) Physical principles of electron microscopy : an introduction to TEM, SEM, and AEM. Springer, 202.
- Clarke, A. R. (2002) Microscopy techniques for materials science. CRC Press (electronic resource)
Related Links
- Petroglyph--An atlas of images using electron microscope, backscattered electron images, element maps, energy dispersive x-ray spectra, and petrographic microscope-- Eric Chrisensen, Brigham Young University
Teaching Activities
- Argast, Anne and Tennis, Clarence F., III, 2004, A web resource for the study of alkali feldspars and perthitic textures using light microscopy, scanning electron microscopy and energy dispersive X-ray spectroscopy, Journal of Geoscience Education 52, no. 3, p. 213-217.
Energy Dispersive X-ray Spectroscopy (eds) Pdf

Energy Dispersive X-ray Spectroscopy (EDS or EDX) is a qualitative and quantitative X-ray microanalytical technique that provides information on the chemical composition of a sample for elements with atomic number (Z) >3.
Characteristic X-ray Generation
The atoms are ionized by the primary electron beam leading to holes generated on the core shells; following ionization the electrons from outer shells fill the holes and cause the emission of X-ray fluorescence lines.
The characteristic X-ray lines are named according to the shell in which the initial vacancy occurs and the shell from which an electron drops to fill that vacancy.

- When used in 'spot' mode, a user can acquire a full elemental spectrum in only a few seconds. Supporting software makes it possible to readily identify peaks, which makes EDS a great survey tool to quickly identify unknown phases prior to quantitative analysis.
- EDS can be used in semi-quantitative mode to determine chemical composition by peak-height ratio relative to a standard.
Energy-Dispersive X-Ray Spectroscopy. Energy-dispersive X-ray spectroscopy (EDX) is a surface analytical technique where an electron beam hits the sample, exciting an electron in an inner shell, causing its ejection and the formation of an electron hole in the electronic structure of the element. EDS - Energy Dispersive X-Ray Spectroscopy from the Technology Data Exchange - Linked to trusted TDE listed vendors.
- The subject of this project is about 'Energy Dispersive X-Ray Fluorescence ' (EDXRF). This technique can be used for a tremendous variety of elemental analysis applications. It provides one of the simplest, most accurate and most economic analytical methods for the.
- Energy-dispersive X-ray spectroscopy (EDX or EDS) is an analytical technique used to probe the composition of a solid materials. Several variants exist, but the all rely on exciting electrons near the nucleus, causing more distant electrons to drop energy levels to fill the resulting 'holes.'.
- Dispersive X-ray spectroscopy (WDS or WDX) separates the X-rays by diffracting them with crystals, collecting one wavelength, or energy, at a time. In contrast, its sister technique, energy- dispersive X-ray spectroscopy (EDS or EDX), collects X-rays of all energies simultaneously. The two methods are almost always used in combination.
Limitations
- There are energy peak overlaps among different elements, particularly those corresponding to x-rays generated by emission from different energy-level shells (K, L and M) in different elements. For example, there are close overlaps of Mn-Kα and Cr-Kβ, or Ti-Kα and various L lines in Ba. Particularly at higher energies, individual peaks may correspond to several different elements; in this case, the user can apply deconvolution methods to try peak separation, or simply consider which elements make 'most sense' given the known context of the sample.
- Because the wavelength-dispersive (WDS) method is more precise and capable of detecting lower elemental abundances, EDS is less commonly used for actual chemical analysis although improvements in detector resolution make EDS a reliable and precise alternative.
- EDS cannot detect the lightest elements, typically below the atomic number of Na for detectors equipped with a Be window. Polymer-based thin windows allow for detection of light elements, depending on the instrument and operating conditions.
Results
A typical EDS spectrum is portrayed as a plot of x-ray counts vs. energy (in keV). Energy peaks correspond to the various elements in the sample. Generally they are narrow and readily resolved, but many elements yield multiple peaks. For example, iron commonly shows strong Kα and Kβ peaks. Elements in low abundance will generate x-ray peaks that may not be resolvable from the background radiation.
Energy Dispersive X-ray Spectroscopy Pdf
References
- Severin, Kenneth P., 2004, Energy Dispersive Spectrometry of Common Rock Forming Minerals. Kluwer Academic Publishers, 225 p.--Highly recommended reference book of representative EDS spectra of the rock-forming minerals, as well as practical tips for spectral acquisition and interpretation.
- Goldstein, J. (2003) Scanning electron microscopy and x-ray microanalysis. Kluwer Adacemic/Plenum Pulbishers, 689 p.
- Reimer, L. (1998) Scanning electron microscopy : physics of image formation and microanalysis. Springer, 527 p.
- Egerton, R. F. (2005) Physical principles of electron microscopy : an introduction to TEM, SEM, and AEM. Springer, 202.
- Clarke, A. R. (2002) Microscopy techniques for materials science. CRC Press (electronic resource)
Related Links
- Petroglyph--An atlas of images using electron microscope, backscattered electron images, element maps, energy dispersive x-ray spectra, and petrographic microscope-- Eric Chrisensen, Brigham Young University
Teaching Activities
- Argast, Anne and Tennis, Clarence F., III, 2004, A web resource for the study of alkali feldspars and perthitic textures using light microscopy, scanning electron microscopy and energy dispersive X-ray spectroscopy, Journal of Geoscience Education 52, no. 3, p. 213-217.
Energy Dispersive X-ray Spectroscopy (eds) Pdf
Energy Dispersive X-ray Spectroscopy (EDS or EDX) is a qualitative and quantitative X-ray microanalytical technique that provides information on the chemical composition of a sample for elements with atomic number (Z) >3.
Characteristic X-ray Generation
The atoms are ionized by the primary electron beam leading to holes generated on the core shells; following ionization the electrons from outer shells fill the holes and cause the emission of X-ray fluorescence lines.
The characteristic X-ray lines are named according to the shell in which the initial vacancy occurs and the shell from which an electron drops to fill that vacancy.
For instance, if the initial vacancy occurs in the K shell and the vacancy filling electron drops from the adjacent (L) shell, a Kα x-ray is emitted. If the electron drops from the M shell (two shells away), the emitted x-ray is a Kβ x-ray. Similarly, if an L-shell electron is ejected and an electron from the M-shell fills the vacancy, Lα radiation will be emitted.
EDS Detector
The detector is based on a semiconductor device, usually a crystal of silicon. The first detector developed was the lithium-drifted silicon or Si(Li) detector, which is now giving way to the silicon-drift detector or SDD.
A typical EDS detector is composed of
- A collimator to ensure that only X-rays generated from where the primary electron beam interacts with the sample will be collected.
- An electron trap to ensure that X-rays, but no electrons, enter the detector.
A window to isolate the detector crystal, under high vacuum, from the chamber of the microscope. Older windows were composed of Be which did not allow low-energy X-rays (< ~0.9 keV) to pass through it, but more modern windows are composed of polymers that will allow low-energy X-rays (down to ~0.1 keV) to pass.
A semiconductor crystal detector.
Electronics to detect the charge recorded by the detector, convert it to a voltage pulse and pass it to the pulse processor.
Detector Operating Principle
- The energy of the incoming X-ray is dissipated by the creation of a series of electron-hole pairs in the semiconductor crystal.
- A high bias voltage is applied across the crystal and this causes electrons and holes to move to electrodes on opposite sides of the crystal, producing a charge signal which is passed to the pulse processor.
- The size of the signal is proportional to the energy of the incoming X-ray. For a silicon detector, ~3.8 eV is used to generate each electron-hole pair (~2.9 eV for Ge). So for an incoming Ni Kα X-ray of energy 7.477 keV, 1968 electron-hole pairs will be produced, and for an Al Kα X-ray of 1.487 keV, 391 electron-hole pairs will be generated.
- By measuring the amount of current produced by each X-ray photon, the original energy of the X-ray can be calculated. An EDS spectrum is essentially a histogram of the number of X-rays measured at each energy.
